Pharmaceutical Products Registration: the Centralized Procedure
The Overview of the Procedure and Timelines
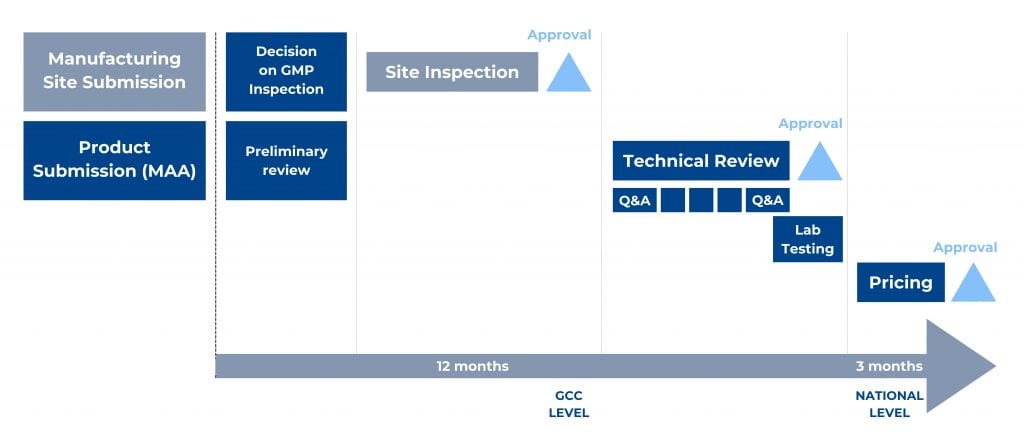 Figure 1. Main steps of the GCC-DR Centralized Procedure and an indicative timeline.
Figure 1. Main steps of the GCC-DR Centralized Procedure and an indicative timeline.
To start the centralized procedure, the Applicant has to submit a registration application for each manufacturing site not accredited by the GCC-DR. In addition, the Applicant should submit the mentioned registration application together with one product marketing authorization application for each desired production line.
A product dossier, prepared as per the GCC CTD format guideline, is first submitted with product samples to the executive office of GCC-DR, reviewed for submission completeness, and dispatched to the member states technical review. Reviews occur separately, then comments from member states are discussed during the upcoming committee meeting. Authorities will notify the Applicant if additional information is required. In such a case, the Committee will review the submitted responses again and again until the provided answers are complete and satisfactory.
Submission in e-CTD format is mandatory since February 2019 and should be done only via the GHC Electronic Gateway. Several documents have been published by GHC, including a guide on “How to Register for the GHC Web Client”, the “GHC Electronic Submission Portal- Naming Convention Files”, and the “Web Client User Guide” to assist applicants.
The region-specific Module 1 has to be prepared according to the “GCC Module 1 Specification and the Baseline eCTD Submission Requirements” guideline of December 2018.
Applicant submits samples, methods, and material for their analysis – which will be done at a laboratory accredited by the Central Committee after issuing a positive opinion on the product application (MAA).
Once the Committee decision is available, the executive office notifies the Applicant and issues the registration certificates for the product and the company/site in case of a favourable opinion. The applicant can appeal the decision within two months from the notification date.












