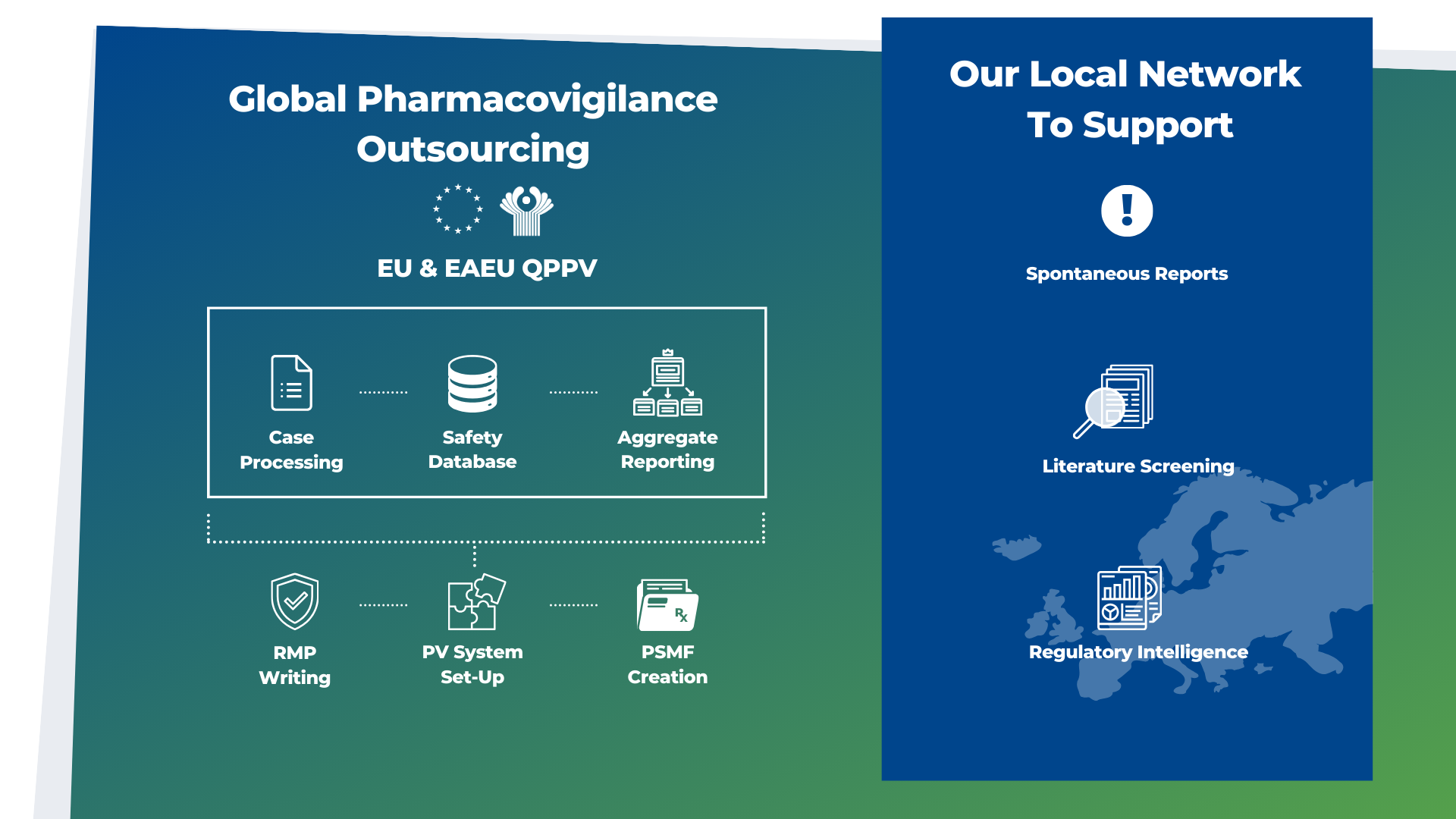
QPPV
Many pharmaceutical firms with a non-EU basis, no in-house PV skills, or business choices to concentrate on key strategic competencies seek to externalize these vital tasks. Therefore, outsourcing QPPV in the EU/EEA has several benefits. Biomapas' QPPV solutions include these professionals in the EU and CIS/EAEU Region to manage and maximise PV outcomes effectively.
Case Processing
An effective pharmacovigilance (PV) program must ensure patient safety at all times. Defined as the identification, collection, and processing of Individual Case Safety Reports (ICSRs), all pharmaceutical and biotechnology firms are required to submit to regulatory authorities following stringent criteria and deadlines. With the help of our PV experts, we can guarantee that you meet your case processing needs.
Safety Database
Biomapas Pharmacovigilance specialists utilize Vault Safety powerful tool for collecting, managing, and providing real-time oversight of adverse events for more informed decisions and improved compliance. In addition, it features streamlined adverse event management with role-based assignment and routing of safety cases for follow-up and medical review.
Aggregate Reporting
Our staff at Biomapas works vigorously to guarantee accurate and compliant aggregate safety reports. As pharmacovigilance and aggregate safety report writers, we have a wealth of experience from pharma and biotech, authorities, and academics. We also provide you with guidance on aggregate safety data analysis and benefit-risk evaluations to ensure patient safety and well-being.
RMP Writing
The Risk Management Plan (RMP) addresses and summarizes a product's safety and efficacy concerns throughout its lifecycle. Biomapas' clinical and quality professionals support you in developing and maintaining a high-quality, compliant RMP.
PV System Set-Up
Post-market monitoring is required in the EU/EEA, the US, and other large pharmaceutical markets (MAA). A comprehensive pharmacovigilance system includes a safety database, SOPs, and a network of trained, certified people working with local authorities in local languages. Thus, a PV system needs significant expenditure, which is not always desirable or practical for smaller pharma. Working together with Biomapas' experts allows you to outsource the complete set-up or individual parts efficiently.
PSMF Creation
A thorough PSMF offers a critical first look into your company's PV system for EU regulatory inspectors. The Pharmacovigilance System Master File is a business-critical document that summarizes the Marketing Authorization Holder's (MAH) PV system. We develop and maintain a PSMF that correctly and completely defines your company's PV system.
Spontaneous Reports
Spontaneous reporting is an important aspect to pharmacovigilance (PV), relying on the motivation of individuals to report suspected adverse drug reactions (ADRs) to a local or national pharmacovigilance centre. Biomapas' spontaneous reporting system handles Individual Case Study Reports (ICSRs) helping you detect and confirm safety signals.
Literature Screening
To maintain compliance with regulatory laws throughout the globe, global and local literature screening is essential to discover reportable Individual Case Safety Reports (ICSRs) and detect new safety signals for developing safety concerns. To guarantee that your pharmacovigilance (PV) program complies with worldwide regulatory standards, our expert team at Biomapas can assist you with all of these tasks.
Regulatory Intelligence
The Regulatory Intelligence services provided by Biomapas may help you establish a sustainable drug development program, perform efficient clinical trials, and develop a solid commercialization strategy. A good plan will save you time and money by combining our expertise with publicly available data and data based on our own experience. We help you make strategic choices and get an advantage in a competitive market.





